
 |
| Estimation of the alkalinity reserves of sugar beet from the ion balance of thick juices. | |
| H. Schiweck and M. Burba, Zuckerindustrie (1993), pp. 241-246 (German). |
 In the summary of this article three equations are given for the calculation of the result of a shortened ion balance from alkalis, αN and invert-sugar (1), the calculation of an extremely shortened ion balance from alkalis and αN and (1a) the calculation of a limit for an equalized ion balance as a difference alkalis minus αN (4): In the summary of this article three equations are given for the calculation of the result of a shortened ion balance from alkalis, αN and invert-sugar (1), the calculation of an extremely shortened ion balance from alkalis and αN and (1a) the calculation of a limit for an equalized ion balance as a difference alkalis minus αN (4):
|
| (1): | w'IB = 0.92 * w'K+Na - (0.57 * w'αN + 1.9 * w'In + 2.9) mmol/100g beet |
| (1a): | w'IB = 0.92 * w'K+Na - 0.57 * w'αN - 4.0 |
| (4): | w'IB,0 for Δw'I = w'K+Na - w'αN = 3.6 ± 0.2 |
|
I cannot understand the meaning of equation (4) and the "very special substitution" and explain that as follows: On page 242, an unclear equation (2a) with two equals signs is given for an equalized ion balance. Unless people use common sense, they might read w'IB,0 = 4.0 from this line: |
|
| (2a): |
w'IB,0 = 0.92 * w'K+Na - 0.57 * w'αN = 4.0 [I would write: 0 = 0.92 * w'K+Na - 0.57 * w'αN - 4.0] |
| Equation (2a) is then transformed by multiplication left and right and by division left and right. The authors jumped directly to the final equation (3) in the journal, but I will write down all steps: | |
| (2a): | 0.92 * w'K+Na - 0.57 * w'αN = 4.0 |
| Multiplication by (w'K+Na - w'αN) left and right: | |
| (0.92 * w'K+Na - 0.57 * w'αN) * (w'K+Na - w'αN) = 4.0 * (w'K+Na - w'αN) | |
| Division by (0.92 * w'K+Na - 0.57 * w'αN) left and right: | |
| (3): | (w'K+Na - w'αN) = 4.0 * (w'K+Na - w'αN) / (0.92 * w'K+Na - 0.57 * w'αN) |
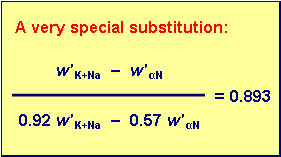
| The very special substitution: | |
| (w'K+Na - w'αN) / (0.92 * w'K+Na - 0.57 * w'αN) = 0.893 | |
|
Introduction of the substitute: |
|
| w'K+Na - w'αN = 4.0 * 0.893 | |
| Final equation of the authors: | |
| (4): | w'K+Na - w'αN = 3.6 ±0.2 |
|
There is only one concentration of alkalis (5.57 mmoles/100 g beet) and one concentration of αN (1.97 mmoles/100 g beet) which fit both equations (2a) and (4). These two equations are given for two unknown concentrations, which is sufficient to find the two concentrations. A reader's assumption that the formulas (2a) and (4) could be universal for an equalized ion balance is wrong. I cannot understand why equation (4) was used to plot three graphs 2-4 and one table, instead of using equations 1a/2a. Equations 1a/2a can be simplified for an equalized ion balance by dividing all terms by 0.92, but the αN cannot end up with a factor of 1 then: (2a) w'K+Na - 0.62 * w'αN = 4.35 NB: The big difference between the alkali factor of this paper and the alkali regression factors found in other papers, including our own, will be discussed on a future page. | |
| 2004-03-14 G. Pollach |
 |
 |